How to report an AEFI or claim compensation
Bagaimana Membuat Laporan AEFI (kesan buruk selepas vaksin) atau Menuntut Pampasan
如何呈报接种后不良反应,以及如何要求赔偿
1. Report via MySejahtera
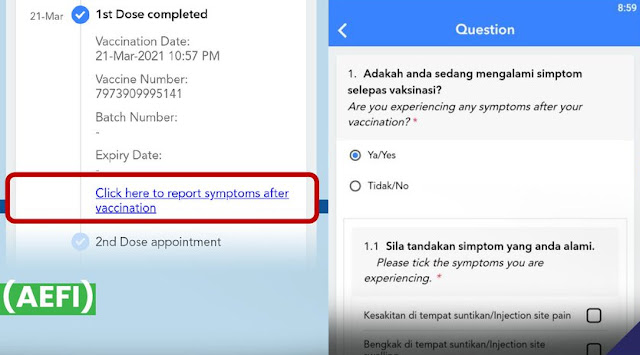
AEFI means "Adverse Effect After Immunisation" (negative side effect). Whether the side effect you experienced is mild or severe, you must report it. You can report a mild side effect on your MySejahtera like this:
Laporkan melalui MySejahtera
AEFI bermaksud Adverse Effect After Immunisation (kesan buruk selepas vaksinasi). Samada kesan buruk yg kamu alami adalah ringan atau berat, kamu perlu melaporkannya. AEFI ringan boleh dilaporkan melalui MySejahtera seperti di atas.
用手机上的 “吾安” 应用程式
AEFI的意思是"免疫接种后的不良反应"(负面的副作用)。无论您经历的副作用是轻微还是严重,您都必须呈报。您可以可以根据以下方法利用 吾安 MySejahtera 来呈报。
2. Report to NPRA yourself
If you don't have MySejahtera or the side effect you experienced is not listed there, report it directly to the NPRA (the National Pharmaceutical Regulatory Agency). The NPRA is an agency under the Ministry of Health that approves medicines and keeps track of adverse reactions (negative side effects). Negative side effects to all medicines and vaccines can be reported here. This form is simple and you can do it yourself:
Consumer Reporting of Side Effects to Medicines or Vaccines
https://npra.gov.my/index.php/en/consumers/reporting/reporting-side-effects-to-medicines-conserf-or-vaccines-aefi-2.html
Laporkan sendiri kepada NPRA
Pelaporan Kesan Sampingan Ubat untuk Pengguna
如果您没有没有手机或 “吾安” 应用程式, 或您经历的副作用未在此处列出,你可以直接向NPRA(国家药品监管机构)投报。NPRA是卫生部下属的一个机构,负责批准药物并跟踪不良反应(负面副作用)。所有药物和疫苗的负面副作用都可以在这里报告。这个表格很简单,你可以自己填写:
https://npra.gov.my/index.php/my/consumers-2/pelaporan/reporting-side-effects-to-medicines-conserf-or-vaccines-aefi.html
3. Serious side effects - Ask your doctor to report to NPRA
If you experienced a serious side effect or a family member died, ask your doctor to report it. The doctor who treated you must fill in this form for you. They can report it online at NPRA's website:
https://www.npra.gov.my/index.php/en/health-professionals/reporting-adr.html
* * *
Kesan sampingan serius - Suruh doktor laporkan kepada NPRA
Kalau kamu kena kesan sampingan serius atau ahli keluarga kamu meninggal, suruh doktor kamu laporkan. Doktor yg merawat kamu perlu membuat laporan ini. Mereka boleh buat laporan secara online di website NPRA:
Laporkan Kesan Advers Ubatan dan Imunisasi (untuk Doktor)
https://www.npra.gov.my/index.php/en/health-professionals/reporting-adr.html
* * *
报告给 KKM
.严重的副作用 - 要求您的医生向 NPRA报告
如果您遇到严重的副作用或家庭成员死亡,请要求您的医生填写报告,治疗您的医生必须为您填写此表格,他们可以在NPRA的网站上在线报告:
Reporting Adverse Drug Reactions and Adverse Events Following Immunisation https://www.npra.gov.my/index.php/en/health-professionals/reporting-adr.html
4. Unofficial report to NGO
You can also make an unofficial report to the NGO below. They keep their own records and statistics because we cannot get any honest info from the MOH. Please note that this is not official and the info DOES NOT go to the Ministry of Health, so it does not matter whether you report here or not. What's important is that you report to the NPRA above. Reporting here helps us to get data that we cannot get from the government. This one is simple and you can do it yourself.
Laporan Kesan Sampingan Selepas Vaksinasi C-19
https://bit.ly/Laporankesansampingan
(You can read the result of all the collected reports here.)
* * *
Laporan tidak rasmi kepada NGO
Kamu juga boleh membuat laporan tidak rasmi dengan NGO di bawah. Mereka mengumpul rekod dan statistik mereka sendiri kerana kita tak boleh mendapat apa-apa maklumat sahih dari KKM. Mereka adalah NGO and laporan kamu TIDAK akan sampai ke KKM melalui portal ini. Yang ni nak lapor atau tidak pun takpa. Yg penting ialah lapor kat NPRA di atas. Laporan di sini membantu kita mendapatkan maklumat yg kita tak boleh dapat dari kerajaan. Borang ini simple dan kamu boleh buat sendiri.
Laporan Kesan Sampingan Selepas Vaksinasi C-19
https://bit.ly/Laporankesansampingan
(Kamu boleh lihat data yg mereka kumpul di sini.)
* * *
您也可以在下面的链接向非政府组织进行非正式的呈报。由于无法从卫生部获得任何诚实的信息,他们只会保留着这些记录和统计数据。 请注意,这不是官方的机构,您呈报的信息不会传到卫生部,因此您是否在此处报告并不重要。最重要的是你一定要根据上面的步骤 1 或 2 向卫生部和 NPRA报告。 这个你可以自己做。
What symptoms should be reported?
Mild symptoms can be reported via MySejahtera. Serious side effects (in the chart below) should be reported to the NPRA.
Examples of serious AEFIs:
Acute allergic reaction or until cannot breathe, baby screams for more than 3 hours, muscle weakness or unresponsive, bacterial infection in blood, abscess at injection site or elsewhere, seizures or seizures from high fever, brain damage, paralysis, nerve pain or damage, blood clots or bleeding under the skin, swollen lymph nodes, bone pain, death, hospitalisation or any other serious illness.
Note: There is no time limit to report deaths or hospitalisations, so don't let anyone fob you off by saying, "Oh, he became seriously ill or died 1 year after vaccination, that's too long already, it's not related." No, it's not. The rules say that there is no time limit, so don't let doctors dismiss you because they are lazy.
Why should you report? So that the NPRA can monitor the safety of a medicine or vaccine. It would help them learn if there are people with certain health conditions which makes them prone to negative reactions to the product. If there are too many bad reactions, it might help them pinpoint a bad batch. Or, if the product is very bad or ineffective, they might withdraw its licensing altogether.
What happens if you don't report it? If the MOH doesn't know that a product is harmful, they will continue to use it. If all the victims before you bothered to report it, maybe this product would not have been given to you and harmed you.
More details in the Garispanduan Farmakovigilans Vaksin Malaysia.
What if your doctor says "Your sickness is not caused by vaccines"? Ask your doctor if they work at the NPRA. No? Then it is not his/her duty to decide whether your ailment is caused by vaccines or not. Their duty is just to fill in the form. You can say something like this: "I'm not asking you to decide whether this is due to the vaccine. I am asking you to report it. The committee at the NPRA will decide if it is due to the vaccine. You don't have to."
If he/she still refuses to do it (because filling in the form is extra work and they are lazy), ask to speak to his/her supervisor or manager. This is your right. They will certainly do it when you say like this, otherwise they will kena goreng.
 |
| Garispanduan Farmakogivilans Keselamatan Vaksin |
AEFI ringan boleh dilaporkan melalui MySejahtera. AEFI yg serius perlu dilaporkan kepada NPRA di atas.
AEFI serius yg perlu dilaporkan:
Kenapa kamu perlu melaporkan kesan buruk? Supaya KKM dapat memantau keselamatan sesuatu ubat atau vaksin. Supaya mereka tahu jika wujud sesuatu golongan pesakit yg tidak sesuai menerima ubat ini dan cenderung kena kesan buruk. Jika terlalu ramai orang yg kena kesan teruk, ia juga boleh membantu KKM mengenalpasti sesuatu batch produk yg rosak. Atau, kalau produk itu terlalu menjejaskan kesihatan atau tidak berkesan, mereka boleh batalkan kelulusan produk itu sama sekali.
Apa akan jadi kalau kamu tak laporkan? KKM tak akan tahu produk ini buruk dan akan terus memberikannya kepada orang lain. Kalau semua mangsa sebelum kamu laporkan, mungkin hari ini kamu tidak akan diberi produk ini dan terkena kesan buruk.
Baca maklumat lanjut di dalam Garispanduan Farmakovigilans Vaksin Malaysia.
Macam mana kalau doktor kata, "Penyakit kamu bukan disebabkan oleh vaksin"? Adakah dia bekerja di NPRA? Tidak? Maka bukan tugas dia untuk memutuskan samada penyakit kamu disebabkan oleh vaksin atau tidak. Tugas dia hanya mengisi borang. Kamu boleh cakap macam ni: "Saya tak minta kamu memutuskan samada penyakit saya disebabkan oleh vaksin. Saya hanya minta kamu buat laporan. Jawatankuasa di NPRA akan menentukan samada ia disebabkan oleh vaksin. Kamu tak perlu buat keputusan apa2."
* * *
轻度症状可以通过吾安手机程式 MySejahtera 呈报。严重的副作用应该向NPRA呈报(如下图)。
更多细节:Garispanduan Farmakovigilans Vaksin Malaysia.
Conditions:
- Malaysian citizen.
- Your AEFI is classed as serious by the Special Covid-19 Vaccine Pharmacovigilance Committee at the NPRA.
- Your AEFI report must be submitted to the NPRA by the doctor who treated your AEFI.
- The side effect must have occurred within 3 months of receiving the vaccine. (If you take longer than that to become sick, you're on your own.)
- For deaths, attach the autopsy report.
- Applications must be submitted within 1 year of the incident.
Send your application to the address below. More details here:
https://citf.mosti.gov.my/faq/en/docs/special-issues/what-is-the-procedure-to-apply-for-financial-compensation-for-severe-covid-19-adverse-effects/
Compensation amount:
Extended hospitalisation: RM50,000.
Permanent disability or death: RM500,000.
条件:
See AEFIs that other people have experienced:
Lihat AEFI yg orang lain kena:
Telegram:
https://t.me/AEFICovidVax
https://t.me/AEFIremaja
Thank you to See Siong for writing the Mandarin translation.
* * * * *


Comments
Post a Comment